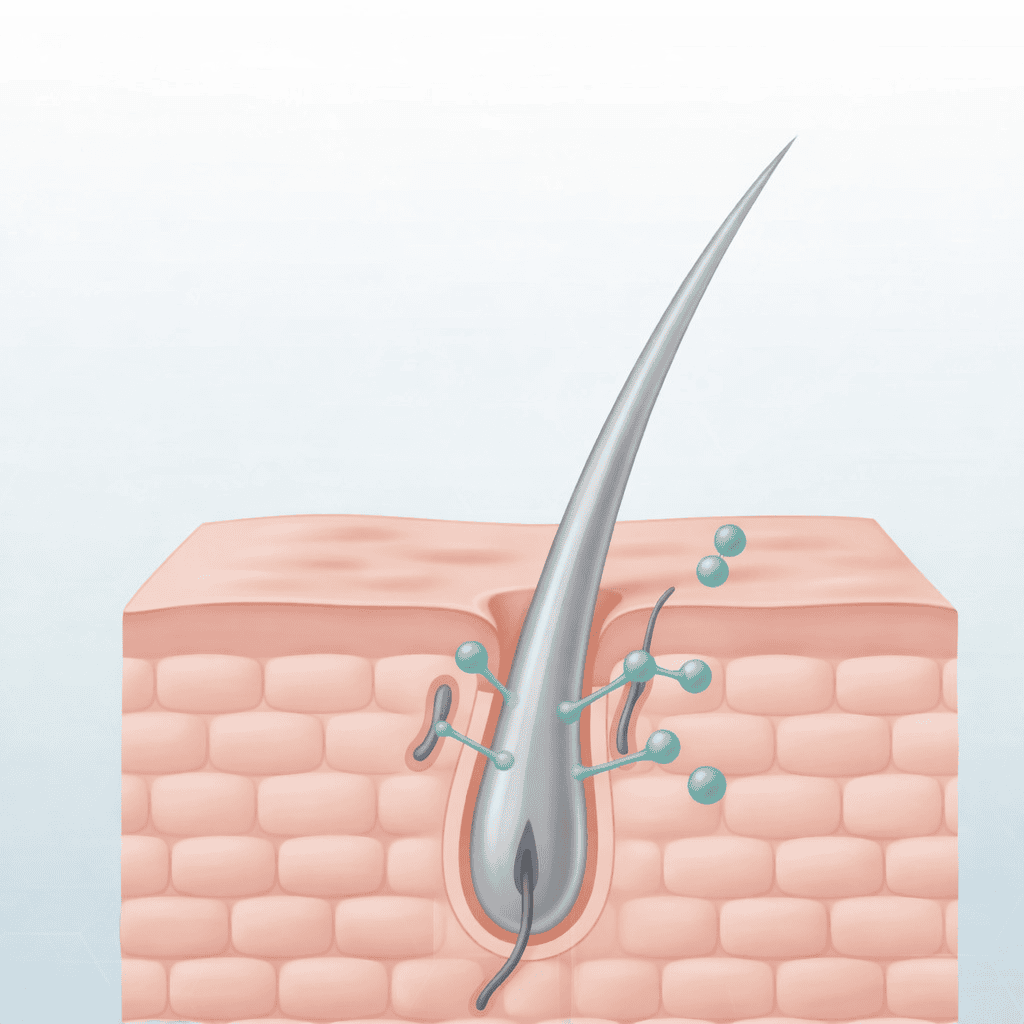
Cosmo Pharmaceuticals has announced encouraging topline findings from two extensive phase 3 trials evaluating the efficacy of clascoterone 5% topical solution in treating male androgenetic alopecia (AGA). This breakthrough could signify the introduction of a new treatment mechanism for AGA, marking the first advancement in over three decades.
The trials, designated as SCALP 1 and SCALP 2, included a total of 1,465 male participants. They measured Target Area Hair Count and collected patient-reported outcomes, revealing statistically significant improvements in hair growth compared to a placebo vehicle. Notably, there was a strong alignment between objective measures and patients’ perceptions of their hair growth.
Clascoterone operates by inhibiting androgen receptors locally at the hair follicle, which minimizes systemic exposure and avoids the hormonal side effects associated with oral treatments. Safety profiles were favorable, with treatment-related adverse events similar to those observed in the vehicle group.
If approved, this therapy could broaden the treatment options available for men seeking a distinctly mechanistic and topical solution for AGA. Regulatory submissions are anticipated following the completion of a 12-month safety follow-up scheduled for Spring 2026.
During the recent Horizons in Advanced Practice meeting held in Tampa, Douglas DiRuggiero, DMSc, MHS, PA-C, presented groundbreaking topical therapies for atopic dermatitis. The therapies discussed included ruxolitinib, roflumilast, and tapinarof.
DiRuggiero pointed out that these newer agents are equally effective as traditional corticosteroids but come with fewer systemic side effects, thereby supporting ongoing efforts in steroid stewardship. Attendees engaged in discussions regarding treatment nuances, age indications, strengths, and insurance-related challenges. Notably, these topical therapies have the potential to significantly replace corticosteroids in maintenance therapy, provided insurance coverage issues can be resolved.
DiRuggiero also underscored the importance of publishing and speaking engagements for PAs and NPs. He argued that sharing case reports and literature reviews contributes to the advancement of the specialty and enhances the broader medical knowledge base.
At the Dermatology Times Horizons in Advanced Practice meeting in Tampa, Florida, conference chairs Lakshi Aldredge, MSN, ANP-BC, DCNP; Omar Noor, MD; and Douglas DiRuggiero, DMSc, MHS, PA-C, led interactive breakout sessions for dermatology NPs and PAs. These sessions focused on complex inflammatory skin diseases, including chronic hand eczema and hidradenitis suppurativa (HS).
Aldredge discussed the evolving landscape of biologic treatments for HS, examining options such as adalimumab, secukinumab, and bimekizumab. She noted that the advent of newer IL-17 targeted therapies has expanded treatment possibilities, even as safety profiles remain comparable and the need for switching due to treatment relapse persists.
Additional discussions addressed adjunctive therapies and the relative ease of obtaining approval for biologics in HS, given the condition’s severity and the limited options available. Aldredge encouraged NPs and PAs to seek opportunities for speaking and publishing, highlighting how mentorship, gradual steps, and resources from professional organizations can foster confidence and scholarly engagement over time.
In a recent custom video series titled Dermatology Times Expert Perspectives, Omar Noor, MD, elaborated on chronic hand eczema (CHE) as a complex, multifactorial inflammatory condition. He emphasized that CHE extends beyond classic type 2 driven atopic dermatitis and often encompasses overlapping phenotypes such as irritant or allergic contact dermatitis, hyperkeratotic disease, and dyshidrotic eczema.
Dr. Noor pointed out that the heterogeneity of CHE involves broader inflammatory signaling through JAK-mediated pathways, advocating for a more comprehensive JAK-targeted treatment approach rather than narrow, cytokine-specific strategies.
Clinically, CHE manifests with a range of variable and often asymmetric symptoms, including fissures, vesicles, hyperkeratosis, and recurrent flares. Assessing severity must take into account not only the dermatological findings but also functional impairment, occupational exposures, chronicity, and responses to prior treatments in order to guide appropriate therapy escalation.
Recludix Pharma has initiated clinical dosing in a first-in-human phase 1 trial for REX-8756 (SAR448755), an oral small-molecule STAT6 inhibitor being developed for type 2 inflammatory diseases. This milestone follows the FDA IND clearance received in December 2025, which also triggered a $20 million milestone payment from their partner, Sanofi.
In preclinical studies, REX-8756 demonstrated rapid and lasting suppression of STAT6 signaling, which is pivotal in the inflammatory processes driven by IL-4 and IL-13. Importantly, it achieves this effect without causing protein degradation, suggesting a biologic-like efficacy in an oral formulation.
The randomized, placebo-controlled phase 1 study aims to enroll approximately 100 healthy volunteers to evaluate safety, tolerability, and pharmacokinetics. This marks a significant advancement in the collaboration between Recludix and Sanofi, which includes potential future milestones worth up to $1.2 billion.